Study: Improving Aortic Aging with Endocalyx Pro
A new paper has been announced by NuLife Sciences and Microvascular Health Solutions, subsidiaries of Bioregenx.


A new paper has been announced by NuLife Sciences and Microvascular Health Solutions, subsidiaries of Bioregenx.
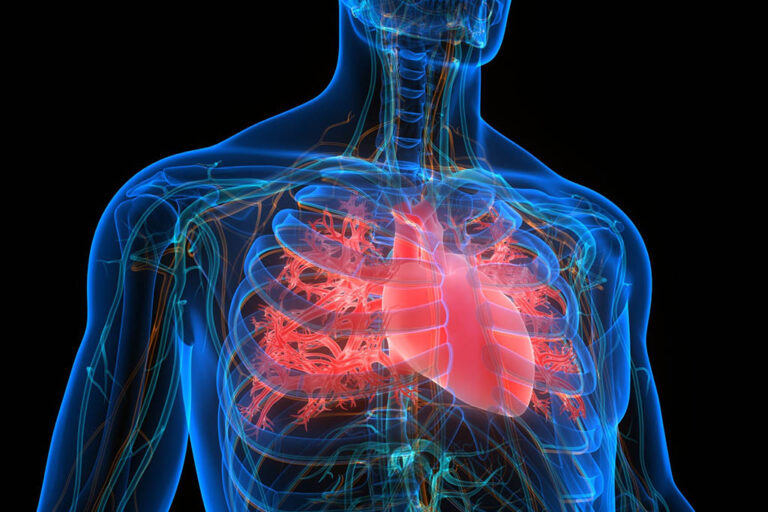
The endothelial glycocalyx is a layer of sugar molecules that coats the inner lining of your blood vessels. It plays a vital role in maintaining vascular health by regulating blood flow, preventing blood clots, and protecting against inflammation.

A study in International Journal of Molecular Science suggests that fucoidan (found in brown seaweed) and supplements containing it may be beneficial to kidney health.

Understanding insulin resistance can be important to understanding your metabolic health. Understanding inositol and how it could affect insulin resistance could be as important.
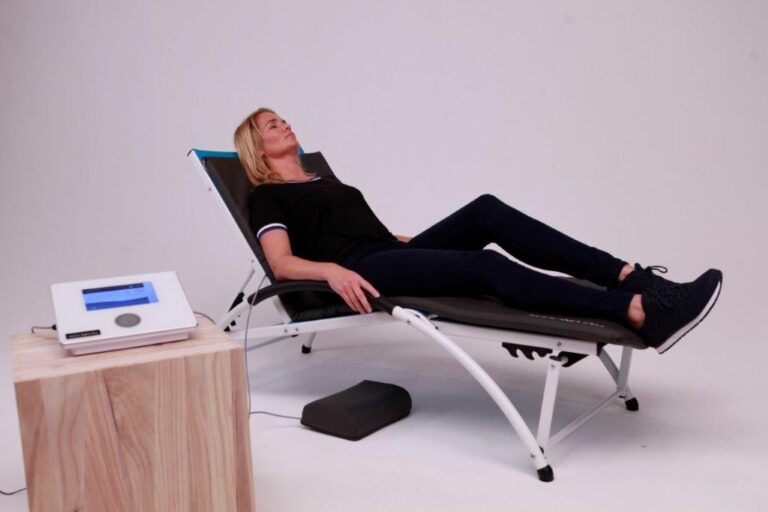
A PEMF therapy device has many benefits and may provide therapy that could potentially work to speed up your recovery from illnesses and injuries as well as to stop or lessen chronic pain.

The authors of the study conclude that the benefits of the Mediterranean-style diet are likely due to improvements in oxidative stress and endothelial cell function.
It took until 50 years ago for the Endothelial Glycocalyx (eGC), a fragile organ that disintegrates at death, to be completely comprehended.

A general rule of thumb is that women over the age of 19 need 310 milligrams a day, and men under 30 years of age need 400 mg a day of magnesium.

Aging gracefully, anti-aging, healthy aging, and longevity – while these may be terms we’ve heard before, navigating what this means and understanding how we can live a longer, better life is half the battle. According to the World Health Organization (WHO), the percentage of us over 60 will almost double by 2050, from 12% to 22%. As we get older, it’s becomes more important to understand a few keys to healthy aging and longevity. We can make minor adjustments to our daily routine to improve our quality of life and, with a little luck, afford us more time to live

Hydrogen Water is Better Than Tap & Bottled Water Simple foods and drinks are often the healthiest. Chemical cocktails like sodas and energy drinks may taste good, but they are terrible for you. An ingredient list longer than a grocery list is a concrete sign of there being something wrong. So, what can someone drink to be healthier? Well, what can be simpler than water and hydrogen? Two vital elements to human bodily function. That’s why hydrogen water has taken off as a health food revolution—as a simple way to improve the body. The only things added are vital minerals
NuLife Sciences, Inc.
7407 Ziegler Rd
Chattanooga, TN 37421
(800) 398-9842

Subscribe to receive exclusive notifications (don’t miss out)
