- Home
- Who We Are
- Shop
- Products
- GlycoCheck
- The Science
- Study: Improving Aortic Aging with Endocalyx Pro
- Improve Vascular Health with This Supplement
- Promising Supplement for Kidney Health
- Inositol & Insulin Resistance
- PEMF Therapy Benefits
- Maximizing Mediterranean Diet Benefits
- Glycocalyx (eGC): What is Endothelial Glycocalyx?
- 9 Tips for a Healthy Aging Lifestyle
- More Science….
- News & Events
- Join
- Login
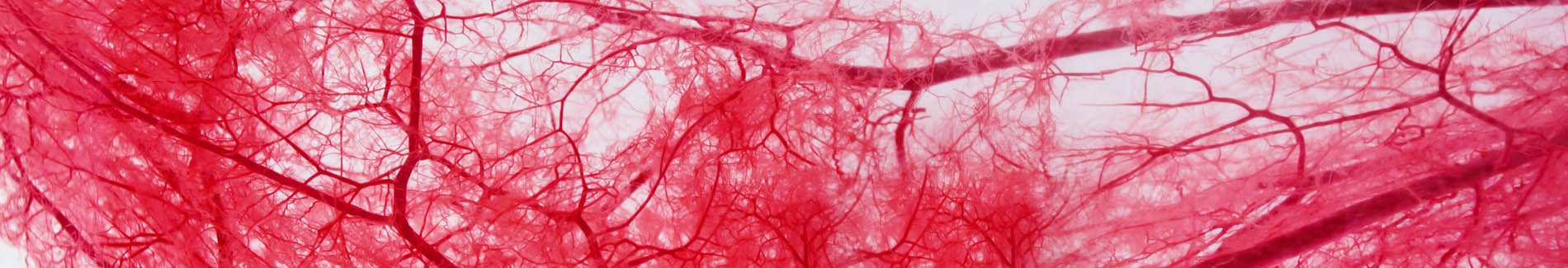
Microvascular Capillaries Before & After Taking Endocalyx Pro*
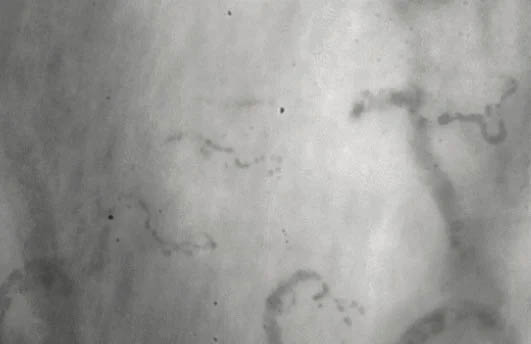
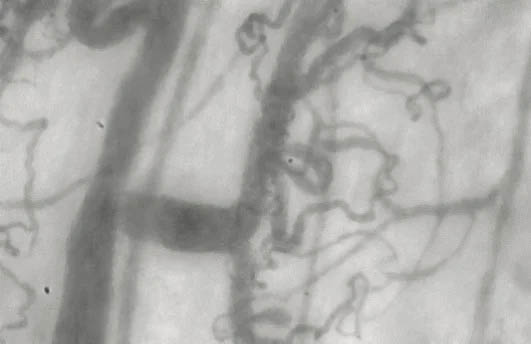
Unhealthy microcirculation (before)
Month 0 – BEFORE taking Endocalyx Pro
Low Microvascular Health Score = 0.6
Healthy microcirculation (after)
Month 4 – AFTER taking Endocalyx Pro
High Microvascular Health Score = 3.9
These before and after images were captured by the GlycoCheck micorvascular testing system. GlycoCheck analyzes capillaries that are as small as 4 microns, so small that about 100 of these tiny capillaries fit inside a human hair.
*Results will vary based on each individual patient. GlycoCheck is a monitoring device and not intend to diagnose any disease.
Endocalyx Pro Vascular Health Supplement
Restore • Regenerate • Protect
Endocalyx Pro is the only proprietary, patented anti-aging supplement that has been clinically shown to maintain a healthy glycocalyx which is a key component of the microcirculatory system and vascular health.*
The glycocalyx is the micro-thin gel layer that lines the inside of all blood vessels throughout the body and all organs.
Endocalyx Pro provides the anti-aging building blocks needed for a strong and vital glycocalyx, strengthening this micro-thin shield that protects the heart, arteries, microcirculation and veins.* It helps keep blood vessels slick, smooth and protected inside, from the largest arteries and veins to the tiniest capillaries.* This keeps blood flowing freely while optimizing circulation throughout the entire body.*

Endocalyx Pro strengthens the glycocalyx and helps optimize the structure of the capillaries, which allows them to exchange oxygen and carbon dioxide, nutrients and waste more efficiently.*
Aging, diet, disease, lack of exercise, genetics, stress and smoking can cause the glycocalyx to become compromised.*
Endocalyx Pro promotes healthy organs associated with vascular health, including the heart, brain, kidneys, lung, muscle, skin and eyes.*
When the glycocalyx is thick and strong, it serves as a barrier to oxidants, fats and cholesterol.*
You can preventatively take Endocalyx Pro, even without being tested for your Micro Vascular Health Score in a healthcare provider’s office.
*Results will vary based on each individual patient. GlycoCheck is a monitoring device and not intend to diagnose any disease.
Science Articles
Quick Navigation
Contact Info
NuLife Sciences, Inc.
7407 Ziegler Rd
Chattanooga, TN 37421
(800) 398-9842